Date: August 21, 2024
Researcher(s): Kyohei Arita
Scientists have determined that the CDCA7 gene is necessary for recognizing and facilitating the repair of partially methylated DNA regions
Mutations in the cell division cycle associated 7 (CDCA7) gene are linked to improper DNA methylation, resulting in disorders like immunodeficiency, centromeric instability, and facial anomalies (ICF) syndrome. Researchers have discovered that CDCA7 plays a crucial role in identifying hemimethylated regions of the DNA and recruiting helicase lymphoid-specific (HELLS) enzymes to facilitate DNA methylation. These findings provide new insights into the underlying disease mechanisms and open new avenues for developing cancer-prevention and anti-aging therapies.
DNA methylation, a process by which methyl groups are added to DNA molecules, is essential for the maintenance of DNA and the overall health of an organism. Disruptions in the standard DNA methylation patterns can lead to immunodeficiency and diseases such as cancer. Helicase lymphoid-specific (HELLS) is an enzyme that facilitates DNA methylation by remodeling the nucleosome - the tightly packed structure of DNA wound around histone proteins. The absence of HELLS or its activator, cell division cycle associated 7 (CDCA7) is known to be a factor that leads to the disruption of DNA methylation. Mutations in the genes that code for HELLS and CDCA7 cause rare disorder immunodeficiency, centromeric instability, and facial anomalies (ICF) syndrome.
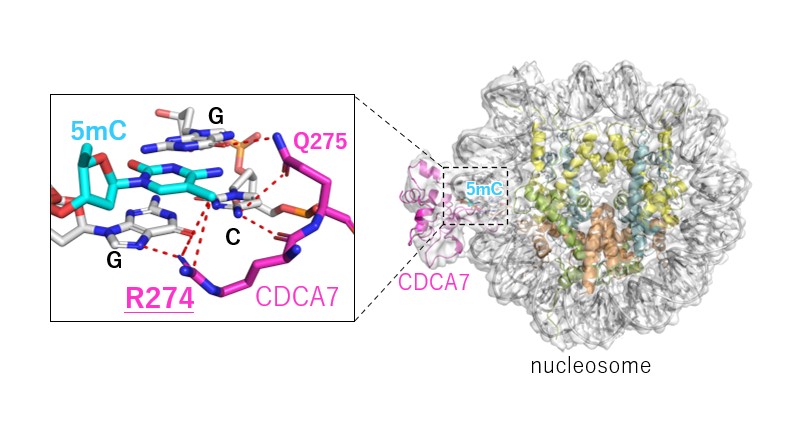
Understanding why CDCA7-HELLS is crucial for maintaining DNA methylation is vital for gaining insights into the mechanism of disorders such as ICF syndrome. In a recent study published in Science Advances on August 23 2024, researchers have found that CDCA7 can recognize hemimethylation of DNA—a state where one strand of the DNA’s double helix is methylated but not the other—and recruit HELLS to complete methylation of the DNA. The researchers found that the hemimethylation-sensing zinc finger (HMZF) of CDCA7, which has been conserved throughout evolution, is key to its ability to perform maintenance DNA methylation. “We found that the CDCA7 gene, known as the causative gene of ICF syndrome, promotes DNA methylation by controlling the ubiquitination of histone H3 through specific binding to hemimethylated DNA on nucleosomes,” explains Associate Professor Atsuya Nishiyama from the Division of Cancer Cell Biology, The Institute of Medical Science, The University of Tokyo, one of the lead researchers of the study.
Dr. Nishiyama, together with Professor Hironori Funabiki and Dr. Isabel Wassing at The Rockefeller University, Professor Kyohei Arita from Yokohama City University and Professor Makoto Nakanishi from The University of Tokyo, examined the structure of the complex formed by CDCA7 and the nucleosome using single particle cryo-electron microscopy. They found that CDCA7, unlike other DNA methylation activators, uniquely identifies hemimethylated DNA in the outward-facing major groove of the nucleosome core particle (NCP). This discovery explains why mutations in CDCA7 associated with ICF syndrome led to a defective DNA maintenance methylation mechanism.
“Our findings suggest that CDCA7 and HELLS promote DNA methylation in a mechanism distinct from de novo DNA methylation, which is now consolidated by our demonstration that the CDCA7 HMZF domain specifically recognizes hemimethylated CpG, the substrate of the maintenance DNA methyltransferase DNMT1. ICF disease-associated mutations in the CDCA7 gene abolish its hemimethylated DNA binding, supporting the functional importance of hemimethylation detection by CDCA7,” Dr. Nishiyama notes.
These pioneering findings not only advance potential therapies for ICF syndrome but also open new frontiers in cancer prevention and anti-aging. Disruptions in DNA methylation is linked to cancer, while inefficient methylation is a hallmark of cellular aging. “Our study lays the groundwork for the development of new DNA methylation inhibitors and therapeutic drugs for ICF syndrome. Therapies that artificially regulate CDCA7-dependent DNA methylation may also prevent cancer and aging and help extend healthy lifespan,” Dr. Nishiyama concludes.
Authors
Isabel E Wassing1, Atsuya Nishiyama2, Reia Shikimachi3, Qingyuan Jia1, Amika Kikuchi3, Moeri Hiruta3, Keita Sugimura2, Xin Hong2, Yoshie Chiba2, Junhui Peng4, Christopher Jenness1, Makoto Nakanishi2, Li Zhao4, Kyohei Arita3, Hironori Funabiki1
Title of original paper
CDCA7 is an evolutionarily conserved hemimethylated DNA sensor in eukaryotes
Journal
Science Advances
DOI
10.1126/sciadv.adp5753
Affiliations
1Laboratory of Chromosome and Cell Biology, The Rockefeller University, New York, NY 10065, USA
2Division of Cancer Cell Biology, The Institute of Medical Science, The University of Tokyo, Tokyo, Tokyo 108-8639 Japan
3Structural Biology Laboratory, Graduate School of Medical Life Science, Yokohama City University, Yokohama, Kanagawa 230-0045, Japan
4Laboratory of Evolutionary Genetics and Genomics, The Rockefeller University, New York, NY 10065, USA
Latest Article Publication Date
23 August, 2024
Method of Research
Experimental study
Subject of Research
Cells
Conflict of Interest (COI) Statement
H.F. is affiliated with Graduate School of Medical Sciences, Weill Cornell Medicine, and the Cell Biology Program at the Sloan Kettering Institute. All authors declare they have no
competing interests.
Dr. Atsuya Nishiyama is an Associate Professor at the Division of Cancer Cell Biology, Institute of Medical Science at The University of Tokyo. Professor Nishiyama has published around 45 scientific articles in areas of cell and cancer research including epigenetics, DNA replication, cell cycle, genome stability, and xenopus proteins. Professor Nishiyama was a former Lecturer at Nagoya City University.
Dr. Kyohei Arita is a Professor in the Department of Science, Graduate School of Medical Life Science at Yokohama City University. He has published extensively on structural bioscience, focusing on DNA methylation, epigenetics, and chromatin biology. His research involves cutting-edge techniques like cryo-electron microscopy and X-ray crystallography. Professor Arita's contributions to the field have been recognized with numerous prestigious awards. In 2023, he received the Yokohama City University President's Award for Excellence, adding to his earlier accolades, including the 2015 Minister of Education, Culture, Sports, Science and Technology Young Scientists Award.