Research
 The primary objective of the Ryo lab is to understand the replication mechanisms of HIV, HBV, HPIV, HTLV and rubella virus. The research goal is to find the weak points of viruses that could lead to the development of novel antiviral drugs and/or vaccines.
The primary objective of the Ryo lab is to understand the replication mechanisms of HIV, HBV, HPIV, HTLV and rubella virus. The research goal is to find the weak points of viruses that could lead to the development of novel antiviral drugs and/or vaccines.
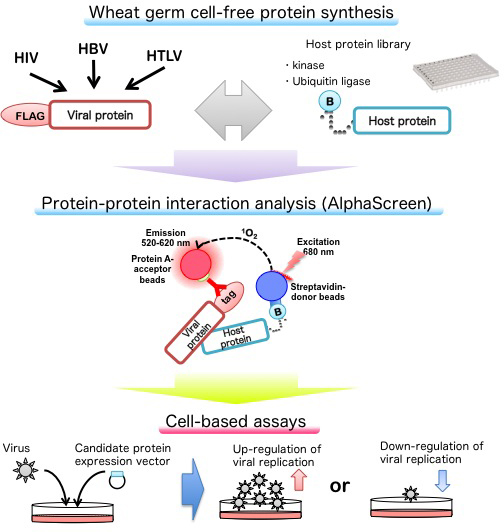
The biochemical research of viruses is often troubled because several viral proteins are hard to synthesize in conventional methods. By using a wheat germ cell-free system, we successfully obtain functional recombinant proteins, which allow us to analyze the biochemical features of viruses.
1. Host – pathogen interaction analysis
The interactions between host factors and viral proteins are essential for viral infection as well as its pathogenesis. We recently focused on the post-translational modifications, such as phosphorylation and ubiquitination, of viral proteins and identified host factors that involve in efficient viral replication. Using in vitro quantitative proteomics, we analyze the host-pathogen interaction network.
References
Kudoh A et al., The phosphorylation of HIV-1 Gag by atypical protein kinase C facilitates viral infectivity by promoting Vpr incorporation into virions. Retrovirology. 22;11:9. 2014
This paper showed that HIV-1 Gag phosphorylation on Ser487 is mediated by aPKC and that this kinase may regulate the incorporation of Vpr into HIV-1 virions and thereby supports virus infectivity. Furthermore, aPKC inhibition efficiently suppresses HIV-1 infectivity in macrophages. aPKC may therefore be an intriguing therapeutic target for HIV-1 infection.
Miyakawa K et al., Interferon-induced SCYL2 limits release of HIV-1 by triggering PP2A-mediated dephosphorylation of the viral protein Vpu. Sci Signal. 2012 Oct 9;5(245):ra73
This paper showed that SCYL2 serves as a regulatory factor for Vpu, reducing the extent of Vpu phosphorylation and consequently enhancing Tetherin/BST2-mediated viral restriction.
2. Development of novel treatment strategies
2-1. High-throughput antiviral drug screening system
Several viral enzymes (such as HIV protease) synthesized by the wheat germ cell-free system are catalytically active. Based on the finding, we developed high-throughput antiviral drug screening system.
References
Matsunaga S et al., Molecular and enzymatic characterization of XMRV protease by a cell-free proteolytic analysis. J Proteomics. 2012 Aug 3;75(15):4863-73.
We utilized the wheat germ cell-free system to synthesize catalytically active XMRV PR for the identification of potential inhibitors and substrates. By this approach we delineated a molecular link between XMRV PR and human tumor suppressor proteins pointing to a potential role in oncogenesis.
2-2. Neutralization antibodies and vaccines
By immunization of recombinant proteins to mice, we created an array of monoclonal antibodies against viral surface proteins (such as PIV HN) to obtain a valuable clone exhibiting virus neutralization activity.
References
Matsunaga S et al., Wheat germ cell-free system-based production of hemagglutinin-neuraminidase glycoprotein of human parainfluenza virus type 3 for generation and characterization of monoclonal antibody. Front Microbiol. 2014 May 13;5:208
This paper reported the development of monoclonal antibodies against hemagglutinin-neuraminidase (HN) of hPIV3 using full-length HN protein synthesized by the wheat germ cell-free system. Our newly-developed MAbs could thus provide a valuable means to explore hPIV3 infection in human cells.
Senchi K et al., Development of oligomannose-coated liposome-based nasal vaccine against human parainfluenza virus type 3. Front Microbiol. 2013 Nov 26;4:346
This paper provided the first evidence that intranasal co-administration of OML-encapsulated HN with the poly(I:C) adjuvant augments the viral-specific immunity against HPIV3.
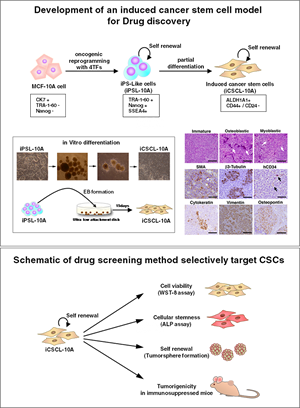
Cancer stem cells (CSCs) play a pivotal role in cancer development, maintenance of tumor tissues, and are likely to be the source of tumor recurrence and metastasis. Thus, CSCs are a promising therapeutic target in cancer treatment. The development of novel therapeutic interventions based on the characterization of CSCs is needed to overcome their resistance to conventional anti-cancer therapies. Unfortunately, the separation and purification of the CSCs from human tumor tissues is complicated by a plethora of technical difficulties that prevent the discovery of new anti-CSC drugs.
We recently developed a method for preparing induced CSC-like cells by oncogenic reprogramming of non-tumorigenic human mammary epithelial MCF-10A cells via the transduction of defined reprogramming factors (Nishi et al, Oncogene. 2014 Jan 30;33(5):643-52). These cells, termed induced cancer stem cell-like 10A (iCSCL-10A), express cancer stem markers (CD44, SOX2 and ALDH1) and show much higher sphere forming ability than conventional cancer cell lines even in regular cell culture media supplemented with fetal bovine serum. Furthermore, only several hundreds of iCSCL-10A cells can form hierarchically organized tumors in immunosuppressed mice in a short period.
Using the iCSCL-10A cells, we developed a phenotypic drug assay system to identify agents that inhibit the stemness and self-renewal properties of CSCs (Nishi et al, Oncotarget. 2014 Sep 30; 5(18):8665-80).
This model is a unique tool not only for analyzing the properties of CSCs but also for screening drugs that selectively target the traits of CSCs (Nishi et al, Cancer Cell & Microenvironment 2014;1:e461). Our approach provides great promise for the future development of novel anti-CSC drugs with the aim of completely curing of cancer.