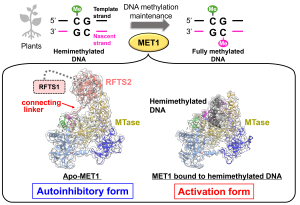
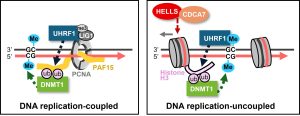
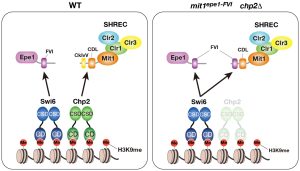
A research paper by Nao Nakamura (M2) and Sae Yoshimi (Master’s graduate, FY2022) has been published in the journal Structure, highlighting new insights into the activation mechanism of the deubiquitinase USP7.
USP7 plays a key role in DNA maintenance methylation by removing ubiquitin from histone H3. In this study, we developed an in vitro assay system to evaluate the deubiquitination activity of USP7 and conducted a series of biochemical experiments to dissect the reaction in detail.
Using cryo-EM, we successfully visualized USP7 in complex with DNMT1, revealing two distinct structural states: an open, inactive conformation and a closed, active conformation. These findings provide a molecular basis for how USP7 becomes activated and highlight the dynamic nature of its function.